Salud Mental 2015;
ISSN: 0185-3325
DOI: 10.17711/SM.0185-3325.2015.012
Received first version: January 28, 2015. Second version: March 17, 2015. Accepted: March 24, 2015.
Psychometric and diagnostic properties of the Drug Abuse Screening Test (DAST): Comparing the DAST-20 vs. the DAST-10.
Luis Villalobos Gallegos 1 , Alejandro Pérez López 1 , Rebeca Mendoza Hassey 2 , Javier Graue Moreno 1 , Rodrigo Marín Navarrete 1
1 Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz (INPRFM), Unidad de Ensayos Clínicos en Adicciones y Salud Mental.
2 Comisión Estatal Contra las Adicciones de Querétaro. Independencia 97, Centro Histórico. Zip code: 76000. Querétaro, Qro.
Correspondence: Rodrigo Marin-Navarrete. Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz (INPRFM), Unidad de Ensayos Clínicos en Adicciones y Salud Mental. Calz. México-Xochimilco 101, San Lorenzo Huipulco, Tlalpan, Zip code 14370, México City; Phone: +52 55 4160-5482. E-mail: rmarin@inprf.gob.mx
Abstract
Background. The use of reliable and valid self-report questionnaires to identify drug use disorders (DUD) is a strategy that has shown usefulness for screening. One of the instruments more used for detection is the Drug Abuse Screening Test (DAST). The psychometric properties in the 20- and 10-item versions have been evaluated in other countries but in Mexico the psychometric and diagnostic properties of both versions are yet to be evaluated.
Objective. The purpose of this study was to evaluate the psychometric and diagnostic properties of DAST-20 and -10.
Method. The sample included 565 participants receiving care in addiction residential centers. The DAST-20 was used as a measure to screen for DUD, and the Mini International Neuropsychiatric Interview 5.0 was used as “gold standard” for the DUD diagnosis. Cronbach’s α and CFA were estimated in order to evaluate the psychometric properties. The Receiver Operator Characteristic (ROC) analysis was used to examine the diagnostic properties of each version.
Results. Both versions obtained a Cronbach’s α ≥ .80, an optimal goodness of fit for the one factor model and Areas Under the Curve ≥ .90 (95% CI 87-93) for both versions.
Discussion and conclusion. DAST-20 and -10 versions are reliable and valid tools for DUD assessment and screening.
Key words: Substance abuse detection, substance-related disorders, psychometrics, ROC curve.
Resumen
Antecedentes. El uso de cuestionarios de autorreporte confiables y válidos para detectar trastornos por consumo de drogas (TCD) es una estrategia que ha mostrado utilidad para detección temprana. Uno de los instrumentos más utilizados para su detección es el Cuestionario de Abuso de Drogas (CAD). Las propiedades psicométricas en su versión de 20 y 10 reactivos han sido evaluadas en otros países, aunque en México no se ha reportado comparación entre las propiedades psicométricas y diagnósticas de ambas versiones.
Objetivo. El propósito de este estudio fue evaluar las propiedades psicométricas y diagnósticas del CAD-20 y CAD-10.
Método. La muestra incluyó 565 personas quienes recibían atención en centros residenciales para la atención de las adicciones. Se utilizó el CAD-20 como medida para la detección de TCD y como “estándar de oro” la Mini Entrevista Neuropsiquiátrica Internacional versión 5.0 para TDC. Se evaluó el α de Cronbach y el Análisis Factorial Confirmatorio para obtener las propiedades psicométricas. También se realizó un análisis de curvas ROC para examinar las propiedades diagnósticas de cada versión.
Resultados. Ambas versiones mostraron un α de Cronbach ≥ .80, excelente ajuste para un modelo unifactorial y un Área Bajo la Curva ≥ .90 (95% CI 87-93) en ambas versiones.
Discusión y conclusión. El CAD-20 y CAD-10 son herramientas confiables y válidas, útiles para detección y evaluación de TCD.
Palabras clave: Detección de abuso de sustancias, trastornos por consumo de sustancias, psicometría, curvas ROC.
Background
The use of self-report questionnaires for the case detection of drug use disorders (DUD) stands as a strategy to decrease the harmful consequences of DUD. This is based on the principle that using reliable and valid screening tools for the early detection of DUD might result in an improvement in the prognosis and a reduction of treatment costs.1 One of the most used instruments to screen for DUD is the Drug Abuse Screening Test (DAST), developed and validated in a sample of patients seeking treatment for substance use problems.2 Although the original version included 28 items, recent studies have focused in analyzing the psychometric properties of 20- and 10-item brief versions of the DAST.
The DAST-202 has been evaluated in various samples including narcotic users,3 workers,4 psychiatric patients5 and burnt patients,6 showing a moderate to good internal consistency ranging between .74-.932,3,5,7 and a test-retest reliability from .78 to .85.4,5 Other studies analyzed the DAST diagnostic accuracy, suggesting that the best cut-off scores to identify drug use problems were between four and six.4,5,6,7,8
On the other hand, the DAST-10 has been evaluated with inpatient substance abusers,9 psychiatric patients5,10 and Latino drug users,11 obtaining a Cronbach’s α that ranged from .86 to .94,10,11 and a test-retest reliability from .71 to .90.5,10,11 Also, these studies suggest that cut-off scores between two and four are more accurate for drug abuse identification.5,7,7,9,10,11,12
In spite of the amount of evidence on the psychome-tric properties of the DAST-20 and DAST-10, a direct comparison of the factorial structure and diagnostic accuracy between both versions is yet to be conducted.
In Mexico, the DAST-20 is widely used at primary care addiction centers to identify problematic substance use.13,14,15 The DAST-20 has been evaluated in Mexican DUD outpatients, reporting a test-retest reliability of .98, an internal consistency of .96 and a concurrent validity with DSM-IV diagnostic criteria for drug abuse and dependence.16 The psychometric properties of the DAST-10 have been assessed in a sample of high school students from Mexico City.17 In spite of these studies, to our knowledge there is no evidence on the DAST diagnostic accuracy to screen drug use disorders in Mexican population.
Objective
This study is to evaluate the psychometric properties and diagnostic accuracy of both the DAST-20 and DAST-10 in a sample of patients from addiction residential care centers in Mexico.
Method
This study is a secondary analysis from a multisite cross sectional study on psychiatric disorders in a sample of inpatients diagnosed with sustance use disorders (SUD) implemented within the Clinical Trials Network on Addiction and Mental Health of the National Institute of Psychiatry Ramon de la Fuente Muñiz (REC-INPRFM). Data were collected between September and November 2013 at 30 addiction residential care centers in the states of Mexico, Puebla, Queretaro and Hidalgo, and Mexico City.
Participants
Participant inclusion criteria were: being between 18-60 years of age; literate; admitted to the center to treat substance use problems; and having at least one week of abstinence. Exclusion criteria were: showing symptoms of psychosis, mania, hypomania or cognitive impairment during screening.
Measures
The Mini International Neuropsychiatric Interview 5.0 (MINI 5.0) in Spanish is a structured diagnostic interview that explores symptoms for Axis 1 psychiatric disorders according to the American Psychiatry Association´s Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) and the International Classification of Diseases 10th Revision (ICD-10). The reliability and validity of this questionnaire are presented elsewhere.18 Results from the MINI 5.0 were used as a “gold standard” for DUD diagnosis.
The Drug Abuse Screening Test is a 20-item self-report questionnaire that assesses the extent of the problems related to drug misuse, using two response options in each item (yes-no). The DAST total score is computed by summing all items; thus, the total score might range from 0 to 20. For this study, version of the DAST previously adapted for the Mexican population was used.16
Procedures
All subjects were recruited for voluntary participation at each center and were assessed for eligibility using the Mini-Mental State Examination (MMSE) in Spanish for cognitive impairment19 and the MINI 5.020 to assess substance abuse/dependence, as well as other psychiatric conditions. The DAST was administered to all eligible participants after screening. All study procedures were approved by the Institutional Review Board of the National Institute of Psychiatry Ramon de la Fuente Muñiz. All participants provided a written informed consent before study participation.
Interviewer training
A team of five interviewers and a field supervisor, all with experience in addiction treatment, from the local institutes and councils against addictions were selected to conduct all study procedures. All team members went through a training and certification process on study assessments and procedures conducted by two experts (a psychiatrist and a clinical psychologist) from the Clinical Trials Unit at the National Institute of Psychiatry Ramon de la Fuente Muñiz.
Data analysis
Internal consistency was evaluated using an alpha coefficient. Confirmatory Factor Analysis (CFA) was conducted to test the one- and two-factor models suggested by Cocco and Carey5 in the DAST-20 and the one-factor model proposed for the DAST-10. Chi square tests (χ2), Degrees of Freedom (df), Comparative Fit Index (CFI), Root-Mean-Square Error of Approximation (RMSEA) and Tucker-Lewis Index (TLI) were estimated taking into account current recommendations for reporting CFA studies21 using Mplus 622 with weighted least squares with mean and variance adjusted estimation and delta parametrization. Cut-off scores for both versions were estimated with a Receiver Operator Characteristic (ROC) analysis, using DUD diagnosis obtained with the MINI-Plus 5. To compare the DAST-20 and DAST-10 areas under the curve (AUC), a DeLong´s test was conducted using pROC23 and epiR24 packages of the R software.
Results
Participants
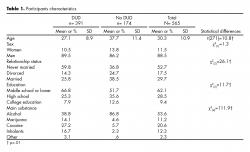
Data from a total of 565 participants were analyzed for this study. There was a significant difference in the age between participants with DUD and no DUD. Most of them reported not being married and living in urban areas. Level of education varied significantly between genders ( table 1). Mini-Plus 5 identified 322 males and 37 females with DUD in the sample, with no statistical difference between genders in DUD prevalence (χ2(1)=1.38, p> .05).
Reliability and validity
The mean score for DAST-20 was 10.67 (SD=5.64). Resulting Cronbach’s α= was .89 (95% CI .88-.91) and item-total significant correlations were above .40, excepting the items 4 and 5, which obtained correlations of -.10 and -.32, respectively. Meanwhile, the DAST-10 mean score was 5.44 (SD= 2.91) with a Cronbach’s α=.80 (95% CI .78-.82).
Regarding CFA, the DAST-20 two-factor model (correlating items 1 and 3) resulted in: χ2=592.4, df=168, CFI=.97, RMSEA=.06 (90% CI .06-.07) and TLI=.97. A single factor model (correlating item 1 with 2 and 1 with 3) resulted in a χ2=645.0, df=168, CFI=.97, RMSEA=.07 (90% CI .08-.07) and TLI=.97. For DAST-10, a single factor model (correlating items 1 and 3) was obtained: χ2=77.3, df=34, CFI=.99, RMSEA=.04 (90% CI .03-.06), TLI=.99, indicating a good fit for this model.
Diagnostic accuracy
The ROC analysis showed that the DAST-10 accounted for 90% of the AUC (95% CI .87, .93) and the DAST-20 accounted for 90% of the UAC (95% CI .87, .93), taking into account that a significant score for AUC is >.70, which allows for the assumption that both versions are accurate for DUD detection ( figure 1).
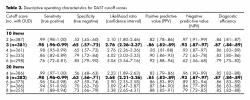
In addition, ROC analysis showed that a cut-off score of 3 for DAST-10 identified up to 98% of the patients with DUD, while a cut-off score of 5 for DAST-20 identified the same proportion of DUD patients ( table 2). No statistically differences were found between DAST-20 and DAST-10 AUC (Z=-.221, p=.82).
Discussion and conclusion
The present study aimed at reporting the psychometric properties and the diagnostic accuracy of the DAST-20 and DAST-10. Both versions obtained a Cronbach’s α>.80, suggesting an equivalent internal consistency. Also, it was found that the one-factor model showed a good fit in both versions. Likewise, both versions predicted an equivalent AUC in the ROC analysis.
Study results were consistent with previous findings in studies conducted with samples from the United States,3,10 India,5 Turkey25 and Korea,26 which may suggest that the internal consistency of the DAST-20 and DAST-10 might be equivalent across different languages.
Another important finding concerns the factorial structure, as the one-factor model obtained adequate fit indexes for both versions; that is: RMSEA <.05, CFI >.95, TLI >.95,27 which makes our results consistent with findings from the Korean and Turkish studies,25,26 but discordant with an Indian study performed in psychiatric outpatients.10 However, the latter study reports little information on CFA procedures (neither indicates the software or estimation method) thus limiting the comparability with this study.
As the DAST measures drug abuse and dependence,3 results support the unidimensionality of the construct, adding to the body of evidence supporting the combination of the biaxial concept of abuse and dependence into a unique entity.28,29,30 Taking into account that the CFA is an analytical approach with a theory driven basis, these results point out to the goodness of fit of the theoretical model established a priori, which in this case is a one factor model.
On the other hand, the highest sensibility was obtained when the cut-off score was 5 for the DAST 20 and 3 for the DAST-10, both with a diagnostic efficiency equal to 97%. This is the suggested score to minimize false negatives.
Regarding the comparison of the results obtained with the DAST-20 and the DAST-10, it is important to note the equivalence in psychometric and diagnostic properties, which may indicate that half of the DAST-20 items are only adding a measurement error, and thus their usefulness might be limited. An obvious advantage of the DAST-10 is its length, considering that a shorter measure not compromised in its psychometric and diagnostic properties results in an optimal tool.
As the DAST properties were partially evaluated in Mexico, this study extends our previous knowledge of its properties using more complex methods, as the DAST is one of the more widely used questionnaires to screen in outpatient settings. Also, as more than a half of the drug users in our country seek treatment in residential centers,31 this study might extend the use of the DAST as a measure of severity and as a strategy to discriminate experimental drug users who do not require residential treatment from patients with DUD who might benefit from such interventions.
The first limitation of this study was that the number of women in the sample was not enough to test the factorial invariance, as the equivalence of the one-factor model of the DAST between males and females is yet to be determined. A second limitation was the absence of a control group of non-clinical population to evaluate the questionnaire capability to differentiate both populations and to obtain specific cut-off scores for non-clinical samples. A third limitation relates to the characteristics of the studied sample (all participants were residential patients). The prevalence of DUD and psychiatric disorders32 is quite high, limiting the applicability of the proposed cut-off scores to screen for DUD in alcohol or drug users with unknown severity.
Overall, the DAST-20 and DAST-10 are useful, reliable and valid tools to screen any DUD. Its use may improve patient identification and referral to specialized treatment. Evaluating its psychometric and diagnostic properties in other populations is needed to determinate applicability in broader contexts.
Funding
This study is part of the project Development of a Clinical Trial Network on Addiction and Mental Health in Mexico funded by a grant from the U.S. Department of State (Grant No. SINLEC11GR0015/A) awarded to the National Institute of Psychiatry in Mexico Ramón de la Fuente Muñiz. The U.S. Department of State had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit this paper for publication.
Conflict of interest
No author of this paper has a conflict of interest, including specific financial interests, relationships, and/or affiliations relevant to the subject matter included in this manuscript.
Acknowledgements
The authors would like to thank the institutional support received by: Instituto para la Atención y Prevención de las Adicciones en la Ciudad de México, Instituto Mexiquense Contra las Adicciones, Consejo Estatal Contra las Adicciones de Hidalgo, Consejo Estatal Contra las Adicciones de Puebla, Consejo Estatal Contra las Adicciones de Querétaro, and the Florida Node Alliance at the University of Miami. We also wish to thank David Sheehan, M.D., for allowing the use of the Spanish-language adaptation of the 5th version of the Mini International Neuropsychiatric Interview in Spanish.
REFERENCIAS
1. Tiet QQ, Finney JW, Moos RH. Screening psychiatric patients for illicit drug use disorders and problems. Clin Psychol Rev 2008; 28: 578-591. doi: 10.1016/j.cpr.2007.08.002
2. Skinner H. The Drug Abuse Screening Test. Addict Behav 1982; 7:363–371.
3. Skinner H, Goldberg A. Evidence for a drug dependence syndrome among narcotic users. Br J Addict 1986; 81:479–484.
4. El-Bassel N, Schilling R, Schinke S, Orlandi M et al. Assessing the utility of the Drug Abuse Screening Test in the workplace. Res Social Work Prac 1997; 7:99–114.
5. Cocco K, Carey K. Psychometric properties of the Drug Abuse Screening Test in psychiatric outpatients. Psychol Assessment 1998; 10:408-414.
6. Salehi SH, As’adi K, Musavi J, Ahrari F et al. Assessment of Substances Abuse in Burn Patients by Using Drug Abuse Screening Test. Acta Med Iran 2012; 50(4):257-264.
7. Pérez B, García L, de Vicente MP, Oliveras MA et al. Validación española del Drug Abuse Screening Test (DAST-20 y DAST-10). Salud Drogas 2010; 10(1):35-50.
8. Gavin DR, Ross HE, Skinner HA. Diagnostic validity of the Drug Abuse Screening Test in the assessment of DSM-III drug disorders. Brit J Addict 1989; 84(3):301-307.
9. Bohn MJ, Babor TF, Kranzler HR. Validity of the Drug Abuse Screening Test (DAST-10) in inpatient substance abusers: Problems of drug dependence. Vol. 119. Proceedings of the 53rd Annual Scientific Meeting, The Committee on Problems of Drug Dependence, Inc., DHHS Publication No. (ADM) 92-1888 NIDA Research Monograph. Rockville, MD7: Department of Health and Human Services; 1991.
10. Carey K, Carey M, Chandra P. Psychometric evaluation of the Alcohol Use Disorders Identification Test and short Drug Abuse Screening Test with psychiatric patients in India. J Clin Psychiat 2003; 64:767-774.
11. Bedregal LE, Sobell LC, Sobell MB, Simco E. Psychometric characteristics of a Spanish version of the DAST-10 and the RAGS. Addict Behav 2006; 31(2):309-319.
12. Maisto SA, Carey MP, Carey KB, Gordon CM et al. Use of the AUDIT and the DAST-10 to identify alcohol and drug use disorders among adults with a severe and persistent mental illness. Psychol Assess 2000; 12(2): 186-192. doi: 10.1037//I040-3590.12.2.186
13. Barragán L, Flores M, Morales S, González J et al. Programa de satisfactores cotidianos para usuarios con dependencia a sustancias adictivas: Manual del terapeuta. México D.F.: Secretaría de Salud/Centro Nacional para la Prevención y el Control de las Adicciones; 2012.
14. Oropeza R, Fukushima EA, García LR, Escobedo JJ. Manual de aplicación del tratamiento breve para usuarios de cocaína (TBUC) (3 ed.). México D.F.: Secretaría de Salud/Centro Nacional para la Prevención y el Control de las Adicciones; 2012.
15. Secretaría de Salud. Modelo de Atención de las UNEME-CAPA “Centros Nueva Vida”. México D.F.: Secretaría de Salud/Centro Nacional para la Prevención y el Control de las Adicciones; 2012.
16. De las Fuentes ME, Villalpando J. Adaptación de un instrumento de tamizaje para población mexicana que consume drogas. [Tesis de Licenciatura]. México DF: UNAM; 2001.
17. Gómez-Maqueo EL, Gómez HL, Morales B, Pérez M. Uso del AUDIT y el DAST-10 para la identificación de abuso de sustancias psicoactivas y alcohol en adolescentes. Revista Colombiana Psicología, 2009; 18(1):9-17.
18. Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Janavs J et al. Reliability and Validity of the M.I.N.I. International Neuropsychiatric Interview (M.I.N.I.): According to the SCID-P. Eur Psychiatry 1997; 12:232-241.
19. Reyes S, Beaman PE, García-Peña C, Villa MA et al. Validation of a modified version of the Mini-Mental State Examination (MMSE) in Spanish. Aging Neuropsychol C 2004; 11:1-11.
20. Ferrando L, Franco-A L, Soto M, Bobes J et al. Mini International Neuropsychiatric Interview. Versión en español 5.0. Instituto IAP; 2000.
21. Jackson DL, Gillaspy JA, Purc-Stephenson R. Reporting practices in confirmatory factor analysis: an overview and some recommendations. Psychol Methods 2009; 14(1):6-23.
22. Muthén LK, Muthén BO. Mplus user’s Guide. Sixth edition. Los Angeles, CA: Muthén & Muthén; 2010.
23. Robin X, Turck N, Hainard A, Tiberti N et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011; 12(1):77.
24. Stevenson M, Nunes T, Sanchez J, Thornton R et al. epiR: Tools for the analysis of epidemiological data. The Comprehensive R Archive Network website. URL http://CRAN.R-project.org/package=epiR. Accessed 2014 September 22. R package version 0.9-59.
25. Evren C, Ogel K, Evren B, Bozkurt M. Psychometric properties of the Turkish versions of the drug use disorders identification Test (DUDIT) and the Drug Abuse Screening Test (DAST-10) in the Prison Setting. J Psychoactive Drugs 2014; 46(2): 140-146, doi: 10.1080/02791072.2014.887162
26. Kim Y. Validating a Korean Version of the Drug Abuse Screening Test-10 (DAST-10). J Soc Serv Res 2014; 40(2):232-241, doi: 10.1080/01488376.2013.875096
27. Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling 1999; 6: 1–55. doi: 10.1080/10705519909540118
28. Becoña E. Trastornos relacionados con sustancias y trastornos adictivos. Revista Iberoamericana Psicosomática 2014; 110:58-61.
29. Hasin DS, O´Brien CP, Auriacombe M, Borges G et al. DSM-5 Criteria for substance use disorders: Recommendations and rationale. Am J Psychiat 2013; 170:834-851.
30. Jones KD, Gill C, Ray S. Review of the proposed DSM-5 substance use disorder. J Addict Offender Coun 2012; 33:115-123.
31. Secretaría de Salud, Centro Nacional para la Prevención de las Adicciones, Comisión Nacional contra las Adicciones, Instituto Nacional de Salud Pública, Instituto Nacional de Psiquiatría. National survey of addictions 2011. México: Secretaría de Salud. Centro Nacional para la Prevención de las Adicciones. Comisión Nacional contra las Adicciones. Instituto Nacional de Salud Pública. Instituto Nacional de Psiquiatría; 2012.
32. Marín-Navarrete R, Eliosa-Hernández A, Lozano-Verduzco I, Turnbull B et al. Estudio sobre la experiencia de hombres atendidos en centros residenciales de ayuda mutua para la atención de las adicciones. Salud mental 2013; 36(5):393-402.