Introduction
Obesity, a global pandemic, has been reported as a potential cause of various diseases, including high blood pressure, type-2 diabetes, cardiovascular disease, cerebrovascular disease, and musculoskeletal disease.1 In addition, obesity is associated with the development of several mental disorders.2
Among the mental disorders caused by obesity are depressive disorder and anxiety disorder. Obesity and depressive symptoms mutually affect each other in a bidirectional manner. Depressive symptoms frequently cause increased appetite and weight,3 while raising the risk of obesity by reducing physical activity.4 Prolonged depressive mood leads to overeating, increasing the risk of weight gain.5 Medicines for treating mood disorder and anxiety disorder can also cause weight gain.6,7 Conversely, obesity can raise the risk of mental diseases,8 particularly in women who are vulnerable to depressive symptoms because of social prejudice against obese women.9 According to a large-scale epidemiology study, the prevalence of depression was four times higher among obese women than among overweight or normalweight women.10 In the case of middle-aged women, reduced estrogen secretion caused by menopause leads to increased body fat, raising the risk of obesity and, subsequently, mental disorders as depression and anxiety.11
Noninvasive brain stimulation is the method to achieve neuromodulation without surgical treatment through the safe local stimulation of a specific area of the brain using magnetism or electricity. Repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (tDCS), and cranial electrotherapy stimulation (CES) are used clinically for the improvement of brain functioning and mental health.12,13 Of these, CES has been approved by US Food and Drug Administration (FDA) as a noninvasive, prescriptive medical treatment for depression, anxiety, and insomnia.14 Kirsch and Nichols (2013) suggested that CES treatment could be very effective for improving the symptoms of depression, anxiety, and insomnia.13 However, it is not clear yet whether CES treatment can induce changes in the plasma indicators that reflect various mood states. Conflicting results have been reported in this regard. For example, Liss and Liss (1996) reported significant increases of tryptophan and endorphins as well as a significant decrease in serum cortisol after CES treatment in both males and females aged between 22 and 70.15 However, in contrast, Lee et al. (2013) reported that CES treatment for preoperative patients resulted in a significant reduction in preoperative anxiety but no significant changes in serum concentrations of stress hormones cortisol and adrenocorticotropic hormone (ACTH).16
In contrast, advanced studies have reported that obesity can cause psychiatric disorders, such as depression, anxiety disorder, schizophrenia, and bipolar disorder. The main factors are hypothalamus–pituitary–adrenal (HPA) axis disturbances, neurotransmitter imbalances, and neuroprogression, along with excessive fat accumulation.3 It has also been reported that a deficiency of brain-derived neurotrophic factor (BDNF), one of the main regulatory factors in neuronal development and synaptic function, is related to the increase in weight.17,18 Thus, the purpose of this study was to examine the effects of regular aerobic exercise and CES on stress hormone levels accompanying HPA axis activation, serum BDNF levels, and changes in mood state among middle-aged obese women.
Method
Subjects
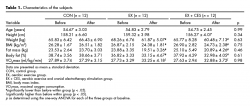
A minimum sample size of 21 participants was deemed necessary for statistical significance, based on a repeated analysis of variance measures, with a 3 × 2 design, an anticipated statistical power of 0.80, an alpha error probability of 0.05, and an effect size of 0.40 (G-power program 3.1.3, Germany). The subjects of this study were 36 middle-aged obese women aged 50 to 59 years who visited the Physical Education Department at Yonsei University in Seoul, Republic of Korea from March 3 to May 30, 2014.
All subjects had to meet the following criteria before enrollment in the research: 1. a BMI above 25kg/m2; 2. no participation in regular physical activity programs; 3. no chronic health problems or smoking; 4. no history of cardiovascular, metabolic, or respiratory disease; and 5. at least 6 months since menopause.
All subjects voluntarily participated in this study and were sufficiently informed of the possible risk factors associated with the experiment. The subjects gave their consent, which included the right to withdraw from the study at any time. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki. Randomization was performed using a simple randomization tool through an Internet site (Random.org) that assigns random numbers by the flip of a coin. The characteristics of the subjects are shown in Table 1.
Anthropometric measurements
Anthropometric measures, which were taken one week before the beginning of interventions, included the measurement of height, body composition, and maximal oxygen consumption (VO2max). Height and body composition were measured using a stadiometer (SECA213; SECA, Hamburg, Germany) and a bio-impedance analysis (BIA) device (Inbody720; Biospace, Seoul, Korea), respectively. VO2max was measured on a treadmill (Q65; Quinton, Seattle, WA, USA) according to the Bruce protocol based on the breath-by-breath method with each subject wearing a gas analyzer (MetaMax 3B; Cortex, Leipzig, Germany). All anthropometric measures were repeated in the same fashion after 8 weeks of intervention to examine changes in the degree of obesity and cardiorespiratory fitness.
Study design
Subjects were randomly assigned to a 12-member control group (CON), an aerobic exercise group (EX), or an aerobic exercise and cranial electrotherapy stimulation (CES) group (EX + CES). Every group received eight weeks of intervention, except for the CON group whose members maintained their own lifestyles with no intervention.
The EX group was subjected to three sessions of 40-minute treadmill running a week; each session included a 10-minutes of warm-up stretching before the exercise and 10-minutes of cool-down stretching after the exercise, according to exercise prescription guidelines for obese subjects.19 Exercise intensity of treadmill running was set at 70% of heart rate reserve (HRR) determined using Karvonen’s formula with the resting heart rate (HRrest) and maximum heart rate (HRmax) calculated during the VO2max test. Heart rate was controlled within a ± 5% range of the target heart rate using a wireless heart rate analyzer (Polar a5; Polar, Finland). The EX group was given identical ear clip electrodes to the EX + CES group, but no electric current was given. The EX + CES group was subjected to the same treadmill running sessions as the EX group, in addition to CES treatment after each session. The CES treatment was administered three times a week using a micro current cranial electrotherapy stimulator (Alpha-Stim 100; Electromedical Products International, Mineral Wells, TX, USA). Based on the Alpha-Stim manual and the previous research of Lee et al. (2013), current and frequency were set at 100 μA and 0.5 Hz, respectively. Clip electrodes were attached to both earlobes to deliver 20 minutes of treatment.16
Blood collection and analysis method
Eight milliliters of blood were collected from the antecubital vein of each subject before and after each intervention using a 22-gauge needle, a serum separator tube (Becton Dickinson, Franklin Lakes, NJ, USA), and an ethylenediamine tetra-acetic acid tube (Becton Dickinson, Franklin Lakes, NJ, USA). Collected blood samples were centrifuged for 15 minutes at 3000 rpm and then stored at -80 °C until analysis.
Plasma stress-related hormone analysis method
The analyses of plasma cortisol were determined by the radioimmunoassay (RIA) using a commercially available Cortisol Coat-A-Count® kit (Cat. no. TKCO1; Siemens, Los Angeles, CA, USA). Similarly, ACTH concentrations were determined by the immunoradiometric assay (IRMA) using a commercially available ACTH IRMA (CT) kit (Cat. no. RE11081; IBL-International, Hamburg, Germany).
Blood neurotrophic factor analysis method
The analyses of serum brain-derived neurotrophic factor (BDNF) were carried out using a human BDNF ELISA Kit (Cat. no. DBD00; R&D Systems, Minneapolis, MN, USA), while nerve growth factor (NGF) analyses used an NGF sandwich ELISA Kit (Cat. no. CYT304; Chemicon, Temecula, CA, USA). A microplate reader (EMax; Molecular device, Sunnyvale, CA, USA) was used to measure absorbance at 450 nm for quantification.
Measurement of mood state
Change in mood state was measured using the Korean edition of the profile of mood states (K-POMS); K-POMS is an adaptation by Kim et al. (2003) of the profile of mood states (POMS) developed by McNair et al. (1992) whose reliability and validity has been verified.20,21 Based on a five-point Likert scale, this questionnaire was composed of a total of 65 questions dealing with six subcategories: Tension-Anxiety, Depression-Dejection, Anger-Hostility, Vigor-Activity, Fatigue-Inertia, and Confusion-Bewilderment.
Statistical analysis
Statistical analyses were performed with the SPSS version 21.0 for Windows (SPSS Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation (SD), unless otherwise stated. A three group (CON, EX, and EX + CES) by time point (before and after intervention) repeated measures ANOVA was used to examine the effect of CES and exercise training on plasma cortisol and ACTH, serum BDNF and NGF, and POMS response. When significant group X time interactions occurred, simple main effects were assessed using one-way ANOVA and paired t-tests. Levels of significance were set at 0.05.
Results
Changes in plasma stress-related hormone levels
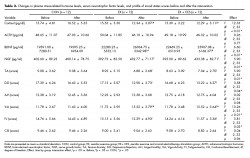
The plasma cortisol and ACTH levels for the three groups before and after intervention are shown in Table 2. Following intervention, repeated ANOVA measures demonstrated a significant difference across group by time interaction for plasma cortisol (F [2, 33] = 12.33, p < .01) levels. Plasma cortisol levels were significantly decreased after intervention when compared with those before intervention in the EX (t = 3.98) and EX + CES (t = 4.86) groups (p < .05). In addition, plasma cortisol levels after intervention were significantly lower in EX and EX + CES groups than in CON group (F [2, 33] = 4.44, p < .05). In contrast, plasma ACTH levels were not significantly different among groups and time points (F [2, 33] = 0.26, p = .77).
Changes in serum neurotrophic factor levels
The serum BDNF and NGF levels for the three groups before and after intervention are shown in Table 2. Following intervention, repeated ANOVA measures demonstrated a significant difference across groups by time interaction for serum BDNF (F [2, 33] = 6.60, p < .01) and NGF (F [2, 33] = 5.90, p = .01) levels. Serum BDNF and NGF levels were significantly increased after intervention when compared with those before intervention in EX (t = -3.84 and t = -4.42, respectively) and EX + CES (t = -4.20 and t = -4.17, respectively) groups (p < .05). In addition, serum BDNF levels after intervention were significantly higher in the EX and EX + CES groups than in the CON group (F [2, 33] = 4.52, p < .05).
Changes in POMS scores
The POMS scores for the three groups before and after intervention are shown in Table 2. Following intervention, repeated ANOVA measures demonstrated a significant difference across groups by time interaction for Tension-Anxiety (F [2, 33] = 5.90, p = .01), Depression-Dejection (F [2, 33] = 3.58, p = .04), Vigor-Activity (F [2, 33] = 13.26, p < .01), and Fatigue-Inertia (F [2, 33] = 3.81, p = .03) scores. Vigor-Activity scores were significantly increased, while Tension-Anxiety, Depression-Dejection, and Fatigue-Inertia scores were significantly decreased after intervention when compared with those before intervention in the EX (t = -6.63, t = 6.66, t = 5.82, and t = 6.43, respectively) and EX + CES (t = -4.57, t = 3.08, t = 5.46, and t = 4.14, respectively) groups (p < .05). In addition, Vigor-Activity scores after intervention were significantly higher in EX and EX + CES groups than in CON group (F [2, 33] = 4.49, p < .05). In contrast, Anger-Hostility and Confusion-Bewilderment scores were not significantly different among all groups and time points (F [2, 33] = .02, p = .98, and F [2, 33] = .04, p = .96, respectively).
Discussion and conclusion
Hypothalamic-pituitary-adrenal (HPA) axis hyperactivation is a characteristic stress response in mammals22 reflected by high plasma cortisol and ACTH levels.23 HPA axis abnormalities are the most common neuroendocrinological disorders found in patients with depression,24 and with other mood disorders.25 It has been reported that 30-50% of the patients with major depressive disorder have evidence of HPA axis dysfunction.24,26 Excessive cortisol secretion in patients with depression was first reported by Board et al. (1956),27 while there have also been reports of significantly higher levels of ACTH secretion in such patients when compared to those in a healthy population.28 The present research analyzed plasma cortisol and ACTH levels to investigate changes in stress-related hormones in obese middle-aged women according to the combined intervention of aerobic exercise and CES. The results show that there was a significant decrease in the plasma cortisol levels after intervention in the EX and EX + CES groups; these decreases were statistically significant when compared to those in the CON group. Such a result is likely due to the alleviation of the HPA axis response by exercise training. This interpretation is supported by the suggestion of Wittert et al. (1996) that although the HPA axis hyperactivation by stress leads to elevated secretion of cortisol, repeated exposure to a particular stress as endurance training could make the HPA axis less responsive to the initial stress.29 Similarly, Fry et al. (1991) reported that exercise training could decrease plasma stress hormone concentrations by gradually improving the stability of the pituitary system.30 Unlike plasma cortisol levels, plasma ACTH levels in the present research did not show significant change following intervention. This result agrees with the findings of Traustadóttir et al. (2005) who investigated the effects of aging and fitness level on HPA axis response in women in their 20’s and 60’s. After exposing them to stress using matt stress reactivity protocol (MSRP),31 he observed no significant difference in ACTH level according to fitness level, although cortisol concentrations were significantly lower in patients with higher than average fitness levels.31 This research led to a similar suggestion that exercise training could alleviate HPA axis response to psychological stress. Similarly, in the present study, based on the significant increase of VO2max after treatment in the EX and EX + CES groups, we believe that the main cause of the changes in plasma cortisol and ACTH levels is the increase in cardiorespiratory fitness due to exercise training.
Neurotrophic factors, such as BDNF and NGF, play crucial roles in regulating the proliferation, migration, survival, and synaptic plasticity of neurons.32 BDNF and NGF are not only involved in the regulation of the neuroendocrine system but can also pass through the blood-brain barrier into the systemic circulation. For this reason, peripheral BDNF and NGF levels have been suggested as biomarkers for diagnosis and disease monitoring in mood disorders such as depression.33,34 For example, based on the meta-analyses of 11 studies, Sen et al. (2008) showed significantly lower BDNF levels in patients with depression than in the healthy control group; this finding suggests that serum BDNF levels could serve as a biomarker for diagnosing psychiatric disorders.33 Similarly, Wiener et al. (2015) reported significantly lower serum NGF levels in patients with major depressive disorder than in a healthy control group.34 The present research analyzed serum BDNF and NGF levels in order to assess changes in neurotrophic factors in obese middle-aged women following aerobic exercise or a combination of aerobic exercise and CES. There were significant increases of serum BDNF and NGF levels after intervention in the EX and EX + CES groups; particularly, serum BDNF levels after intervention were significantly higher in these groups compared to those in the CON group. This result is in agreement with previous studies that concluded that regular aerobic exercise training caused a significant increase of serum BDNF level in obese subjects.35,36 Our findings also support the result of a previous study that found that dietary changes and aerobic exercise training induced significantly higher levels of BDNF and NGF expression in the hippocampus of obese rats.37 In addition, a previous report indicated that dysfunction of BDNF and NGF, as well as of their respective receptors tropomyosin receptor kinase B (TrkB) and tropomyosin receptor kinase A (TrkA), could contribute to the pathogenesis of both depression and obesity.38 Accordingly, the significant increases of serum BDNF and NGF levels in the present research are potentially due to the improvement of obesity by aerobic exercise training. Indeed, after treatment, the EX and EX + CES groups showed significant reductions in body weight, BMI, fat mass, and body fat. This finding is supported by the work of Schulte-Herbrüggen et al. (2009) who reported a negative correlation (r = -0.69, p < .0001) between body weight and serum BDNF levels.38 Similarly, Danaalamdari et al. (2014) reported a negative correlation (r = -0.43, p = .032) between BMI and serum NGF levels.39
Obesity is one of the factors that threatens mental health by causing such abnormal conditions as depression, anxiety, and panic disorders; positive relationships between obesity and such psychiatric disorders have been mainly observed in women.40 The present research utilized the profile of mood states (POMS) to verify the effect of aerobic exercise and the combined intervention of aerobic exercise and CES on the mood state of obese middle-aged women. Following intervention, the EX and EX + CES groups showed significant decreases in Tension-Anxiety, Depression-Dejection, and Fatigue-Inertia scores, as well as a significant increase in the Vigor-Activity score. This result supports prior studies that utilized POMS to demonstrate significant improvement of mood state following aerobic exercise.41,42,43 Specifically, Sakuragi and Sugiyama (2006) reported that four weeks of walking exercise resulted in a significant decrease in the scores in the Tension-Anxiety and Depression-Dejection subscales of POMS.41 According to the report of Petajan et al. (1996), 10 weeks of aerobic training resulted in a significant increase of the Fatigue-Inertia score.42 In addition, Brown et al. (1992) reported that participation in a 9-week aerobic exercise program could bring about significant increase of the Vigor-Activity score.43 The changes in POMS scores in the present research also suggest the positive impact of aerobic exercise training in improving the mood state, and the major contributors to this improvement are thought to be the decrease in cortisol and increases of BDNF and NGF. Such an interpretation is supported by previous reports which point out that regular exercise could be effective for the maintenance and improvement of mental health by reducing distress and negative affect while promoting positive benefits for the cognitive, emotional, and motor domains.44,45 These reports have also suggested that such beneficial effects of exercise could be explained by the maintenance of HPA axis homeostasis, as well as by the elevation of the expression levels of neurotrophic factors such as BDNF and NGF.44,46
On the other hand, there was no significant difference in any variable between the EX and EX + CES groups. Therefore, it is judged that CES treatment did not induce changes in stress-related hormone response, neurotrophic factor activation, and mood state. There have been conflicting results as to whether CES treatment can alleviate and treat such mental diseases as depression and anxiety and induce changes in related biomarkers. For example, Barclay and Barclay (2014) reported that CES treatment was effective for alleviating the symptoms of anxiety and depression,47 but the systematic review of Kavirajan et al. (2014) revealed no positive effect of CES treatment on acute depression.48 In addition, Liss and Liss (1996) suggested that an 18% decrease in plasma cortisol levels after CES treatment was due to the reduced stress response,15 but Lee et al. (2013) reported that CES treatment showed an alleviating effect on preoperative anxiety involving no change in plasma cortisol and ACTH levels.16 There are also conflicting results from a limited amount of research concerning the effect of such noninvasive brain stimulation as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) on neurotrophic factor expression. Zanardini et al. (2006) reported significant increases of serum BDNF after rTMS treatment in drug resistant depressed patients,49 but Brunoni et al. (2014) reported no significant change in BDNF after tDCS treatment.50 Thus, while noninvasive brain stimulation, including CES, can directly regulate the plasticity of the cranial nerves and provide some alleviation and treatment of the symptoms of mental diseases, such as depression and anxiety, patients’ responses to noninvasive brain stimulation could differ according to their individual characteristics (such as the severity of disease, age and gender). There is also a recent report indicating that the efficacies of noninvasive brain stimulation could differ according to the genetic characteristics of patients.51 Moreover, effective timing for applying noninvasive brain stimulation, as well as proper cranial stimulation parameters, still need to be clearly verified.52 Accordingly, follow-up studies would need to verify these parameters using various research techniques (such as factorial, randomized, sham-controlled trial) in consideration of the patients’ diverse characteristics (such as age, gender, and the type and severity of the disease).
In conclusion, our overall results suggest that aerobic exercise training can exert positive effects on the mood state of obese middle-aged women through a decrease in the cortisol levels and increases in BDNF and NGF. However, CES treatment did not have a significant positive effect on mental health status in middle-aged obese women, as the results of CES treatment combined with exercise were not significantly different from those of treatment with exercise alone.
Nevertheless, the present research has some limitations. This was a pilot study conducted at a single organization and therefore had a small number of subjects. In addition, it was previously reported that improvements in mental health status using noninvasive brain stimulation treatment, including CES, can vary according to age, sex, and the type and degree of disease.51 Thus, future studies should recruit a greater number of subjects in order to comprehensively investigate the effects of CES treatment.
Funding
This study was supported by research funds from Dong-A University.
Conflict of interest
The authors declare that there is no conflict of interest.