Background
International reports cite cannabis as the world’s most widely used illegal drug (United Nations Office on Drugs and Crime, 2016). Mexico’s National Addictions Survey not only identifies cannabis as the most widely used illicit drug, but also teenagers’ and young people’s drug of choice after alcohol and tobacco (Villatoro-Velázquez et al., 2016). In addition to epidemiological findings, there is sufficient scientific evidence on the psychiatric consequences of chronic cannabis use and abuse, such as: induced psychotic episodes, affective and anxious symptomatology, cognitive deterioration, amotivational syndrome and dependence (Hall, 2015; Volkow, Baler, Compton & Weiss, 2014), although little is known about other types of medical complications such as Cannabinoid hyperemesis syndrome (CHS) (Contreras-Narváez et al., 2016).
CHS is a medical condition affecting chronic cannabis users, first identified in 2004 (Allen, Moore, Heddle & Twartz, 2004). It is characterized by cyclical episodes of uncontrollable vomiting that may last two to four days; symptoms cannot be explained by medical conditions other than their association with a paradoxical response to cannabis use. This vomiting can be identified by the lack of response to regular antiemetic treatment, as a result of which patients compulsively take hot baths in an attempt to obtain relief. Reports also indicate that vomiting subsides once the patient stops using cannabis and resumes during periods of re-incidence and/or relapse (Allen et al., 2004).
General hospital and emergency room physicians are usually unfamiliar with the syndrome. Moreover, the lack of well-defined standards for CHS diagnosis and treatment increases the number of admissions to emergency rooms and the use of laboratory tests and consultancy studies, which in turn is associated with high costs and malpractice (Galli et al., 2011).
The purpose of this paper is to offer a brief review to inform the medical community of the issue and to report the first documented case of CHS in Mexico.
Method
In order to provide a brief narrative review, a literature search was performed in PUBMED to identify articles on CHS. A specific search algorithm was created that included keywords associated with CHS. A search period from 2004 to 2016 was established. As an inclusion criterion, the authors decided to include all original papers and review articles as well as articles and case reports, and case series available in English and Spanish that complied with CARE case reporting guidelines (Gagnier et al., 2013).
For the registration and documentation of the clinical case presented below, the CARE case reporting guidelines were used (Gagnier et al., 2013).
Results
Brief review
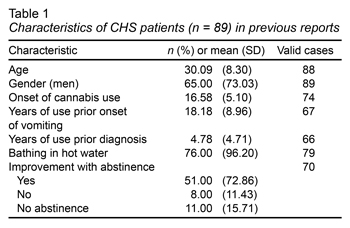
As an overview, since 2004, only 89 cases have been reported worldwide in 46 scientific articles (Contreras-Narváez et al., 2016), although none from Latin American countries. Regarding the characteristics of the 89 patients previously reported, the most outstanding findings were that 65% of the cases were men, with a mean of 30 years. They also presented an average onset of cannabis use of 16 years, and a mean onset of vomiting to 18.18, two years after the age of cannabis initiation. Up to 96.20% of the cases reported using hot-water baths as a remedy and almost 73% reported a significant improvement after cessation of cannabis use (Table 1).
CHS etiology
The etiology of this syndrome is unknown and the available information limited. However, there is evidence of the effects of cannabis on the body, which are regulated by the interaction between exogenous cannabinoids and endocannabinoid receptors (CB1 and CB2) distributed throughout all the systems in the body (central nervous, immune, digestive, gastrointestinal, respiratory, cardiac and reproductive) (Volkow et al., 2014). Moreover, studies have shown that the effects of delta-9-tetrahidrocannabinol (Δ9-THC) on the human body may have therapeutic effects (Duran & Capella, 2004), such as its antiemetic effect to control nausea and vomiting in patients undergoing chemotherapy treatment (Adler & Colbert, 2013).
However, in 2004 the first series of cases was published which identified a paradoxical response was called CHS, and contrary to the usual antiemetic effect of cannabis (Allen et al., 2004).
Although little is known about the etiology, there are three possible theories in the literature:
1. Interaction between Δ9-THC and CB1 receptors at low doses mediates as a partial agonist, creating antiemetic effects, although at high doses it may create hyperemesis (Darmani, 2002; Woods, Wright, Gee & Scobey, 2016).
2. Genetic vulnerability and high doses of cannabis mediate an alteration of the hepatic cytochrome p450 in the metabolization of Δ9-THC, increasing the concentration of the substance in the body (Woods et al., 2016).
3. The high liposolubility of Δ9-THC mediated by over-accumulation in fatty tissue increases the concentration of the substance in the body (Allen et al., 2004).
Diagnostic criteria for CHS
Just as etiological theories, which are as yet inconclusive, diagnostic criteria remain ambiguous and are currently under study. Part of the difficulty of undertaking differential diagnosis is that hyperemesis can be explained by other medical conditions which must be ruled out before establishing the diagnosis of CHS. Conditions associated with vomiting cycles include: cyclical vomiting syndrome, associated with a family history of migraines and psychological stressors; hyperemesis gravidarum, which occurs during pregnancy; psychogenetic vomiting, typically associated with major depression disorder and conversion disorder; bulimia, associated with induced and purgative vomiting; Addison’s disease, associated with chronic fatigue, weight loss, hyperpigmentation, hypotension, hyponatremia and hypercalcemia; and even acute migraine signs (Wallace, Andrews, Garmany & Jelley, 2011). However, only two criteria serve as the gold standard for establishing CHS: chronic cannabis use and compulsive hot bathing (Allen et al., 2004).
Additionally other research groups proposed a categorization of diagnostic criteria: a) Essential: cannabis use for long periods of time (over 12 months), b) Major: Severity of nausea and vomiting, relief through compulsive hot water bathing, periumbilical or epigastric abdominal pain, improvement of symptoms after cessation of cannabis use, and c) Supporting: persons under the age of 50, significant weight loss (five or more kilos) (Patterson et al., 2010; Sontineni, Chaudhary, Sontineni & Lanspa, 2009).
CHS is a recurrent disorder with asymptomatic intervals and it has been proposed to split it into three phases: prodromal, hyperemetic and recovery. During the prodromal phase, the patient may present morning sickness with vomiting and abdominal discomfort for months or years without altering his or her eating habits. During this phase, the patient increases or continues cannabis use, since this relieves nausea. The hyperemetic phase is characterized by paroxysms of nausea and intense, persistent and incapacitating vomiting, with up to five episodes an hour. Most patients present slight, diffuse abdominal distress and the recovery phase begins with the cessation of cannabis use. During the 48 hours following cessation, patients report significant improvement in acute symptomatology, although symptoms recur during periods of re-incidence and relapse (Price, Fisher, Kumar & Hilgerson, 2011).
CHS Treatment
The vast majority of published reports cite hot bathing as the most effective remedy, used by patients to reduce the severe symptoms occurring during the acute stage such as: nausea, vomiting, abdominal discomfort and lack of appetite. Although the exact mechanism is unknown, it has been thought that hot bathing may correct the imbalance caused by cannabis in the thermoregulation system of the hypothalamus (Galli et al., 2011; Sontineni et al., 2009).
Despite the home remedies used by patients to reduce the characteristic discomfort of CHS, the vast majority of cases require hospitalization to access support protocols through intravenous hydration. During endoscopy of the upper digestive tract, patients with CHS often present various degrees of esophagitis and gastritis, which requires routine acid suppression following the administration of proton bomb inhibitors (Galli, Sawaya & Friedenberg, 2011).
There is also evidence indicating the usefulness of drug regimens in counteracting some of the symptoms of acute illness such as: saline solution for rehydration (Allen et al., 2004; Price et al., 2011), morphine for abdominal pain, paracetamol for headaches, lorazepam for anxiety symptomatology, clorpromazina to decrease hiccups, ondansetron to reduce vomiting and nausea; additionally some authors suggest the use of haloperidol as an antiemetic, for its regulatory effects on dopaminergic receptors (Hickey et al., 2013).
Case report
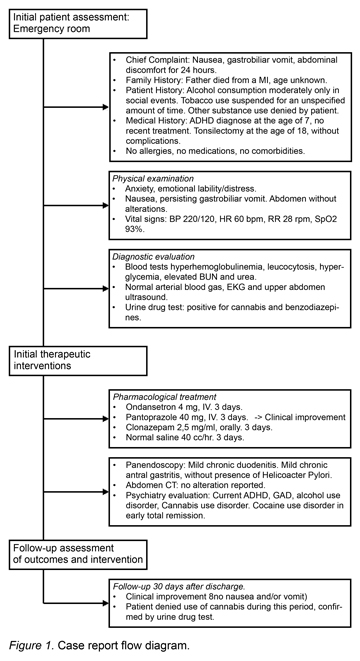
For the registration and documentation of the clinical case presented below, the CARE case reporting guidelines were used (Gagnier et al., 2013) (Figure 1).
This study reports the case of a 37-year old male Mexican patient, with the following medical history:
‒ Family History: Father died from myocardial infarction. Mother alive and well.
‒ Personal/Social History: Moderate alcohol use during social events; no more than four beers per occasion. Tobacco use suspended for an unspecified amount of time. Other substance use denied by patient. No pets, no recent vaccines, no recent trips, no sports activity.
‒ Medical History: ADHD diagnosed at the age of seven, without recent treatment. Tonsilectomy at the age one without complications. Medication use denied by patient. No allergies, no comorbidities.
Chief Complaint
The patient reported that five years ago he began experiencing throughout the day acute symptoms characterized by multiple episodes of nausea, gastrobiliar vomiting and epigastric discomfort, with an approximate duration of 48 to 72 hours. These acute symptoms had occurred previously around three to five times in the last year. Although teams of various medical specialties had studied and treated the patient, none of them had established a specific diagnosis.
On this occasion, the patient was admitted to the emergency department of a private general hospital after presenting epigastric discomfort for the previous 24 hours, and persistent nausea and vomiting beginning earlier that same day.
The algorithm for the diagnosis and treatment provided over a period of three days after the patient’s admission is described below.
Physical exploration
Conscious, oriented, alert. Presenting symptoms of anxiety, emotional lability and distress, presence of constant nausea and gastrobiliar vomiting that hampered proper exploration and neurological exam. Rhythmic heart sounds, clean and well ventilated lung fields. Normoperistaltic abdomen with no tenderness on palpation. Pelvic limbs without edema; with the following vital signs: BP 220/120 mmHg, HR 60 beats per minute, RR 28 respirations per minute, SpO2 93% through pulse oximetry.
Laboratory and imaging studies
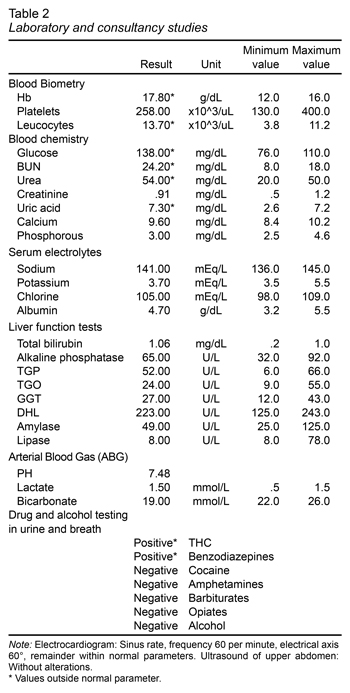
The following laboratory studies were ordered: blood tests, including complete blood count, metabolic panel, liver function tests, arterial blood gas analysis. The tests showed hyperhemoglobulinemia, leukocytosis, hyperglycemia, elevated BUN and urea. The following diagnostic exams were taken with no alterations whatsoever: EKG, liver and biliary tract ultrasound which ruled out biliary disease (Table 1). A psychiatry consultation was requested due to the anxious behavior of the patient, and for a substance use evaluation (Table 2). A urine drug test and alcohol breath test were also ordered, resulting positive for THC and benzodiazepines (Table 1).
Initial treatment
Treatment was initiated with intravenous hydration with normal saline solution, ondansetron 4 mg IV, pantoprazole 40 mg IV and clonazepam 2.5 mg/ml, 3 drops orally. This treatment reduced the patient’s anxiety symptoms and lowered his blood pressure to normal limits. Clinical improvement was achieved approximately 12 hours after hospital admission with a sudden remission of vomiting.
Additional laboratory and imaging studies
During the second day a panendoscopy was performed, revealing a linear ulcer in the gastric antrum with gastroduodenitis following biopsies with a subsequent pathology report describing mild chronic duodenitis and mild chronic antral gastritis without the presence of helicobacter pylori.
Abdominal computed tomography was also performed with oral and intravenous contrast, with an absence of abdominal abnormalities and normal adrenal glands. Twenty-four hour urine was collected to determine the levels of vanyllilmandelic acid, which was reported to be within normal limits (12.3 mg/24 hr), and thus ruled out pheochromocytoma.
Psychiatric and substance use assessment
In order to determine the presence of mental and addictive disorders, a psychiatric assessment evaluation was designed expressly for this purpose, which involved an evaluation of the patient’s mental state by two experts in psychiatry and addictions, with the support of a battery of clinimetric instruments: The Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005), to rule out cognitive impairment; International Neuropsychiatric Interview (Sheehan et al., 1998), to determine possible psychiatric disorders; Time Line Follow Back (Sobell, Sobell, Maisto & Cooper, 1985; Sobell & Sobell, 1992), was applied to determine the pattern of use over the previous 30 days.
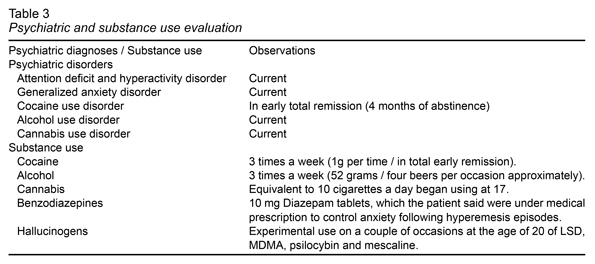
The most significant results of the psychiatric evaluation and patient substance use are reported in Table 3. However, the test revealed the presence of cannabis use disorder. According to the patient, he began using cannabis at the age of 17 and currently smoked the equivalent of 10 cigarettes a day. At the same time, the patient’s attention deficit hyperactivity disorder (ADHD) could be sensitive to the effects of his chronic use. However, he reported that as long as he could remember, he had had problems with concentration and hyperactivity, affecting his academic life from elementary school to the beginning of college, which he interrupted due to ADHD, diagnosed at the age of seven, although it was never treated.
Discharge and follow-up session
After three days of hospital care, the patient was discharged and was given a medical follow-up session after 30 days of discharge. As a significant finding, the patient reported no use of cannabis during that time. As part of the follow-up protocol, a urine test was performed to monitor substance use in the last week and obtained negative results. Additionally the patient did not report any more symptoms of compulsive vomiting and nausea.
Recommendations
Before discharge the following recommendations were made to the patient: a) adherence to a pharmacological treatment composed by antiemetics and hydrogen pump inhibitors, b) to start treatment and rehabilitation for substance use, and c) to maintain total abstinence from cannabis to prevent recurrence of acute CHS.
Discussion and conclusions
It is important to know the toxic effects cannabis causes in the body in both the short and long term. Since Allen et al. first described a report on nine cases of CHS in 2004, several case reports have been published in various parts of the world, which has contributed to a better characterization of the syndrome.
Like the clinical case reported in this paper, previous international reports agree on the presence of acid peptic disease which is common in these patients (perhaps because of their poor eating habits). However, this endoscopic finding should not be considered a gold standard in acute cases of patients with CHS. The presence of acid peptic disease is irrelevant in the broader expression of CHS and creates the risk of misdiagnosis, particularly if the patient is a cannabis user. Also worth mentioning is the abrupt way acute symptoms subside after cessation of cannabis use, including both nausea and vomiting, since these are unique, exclusive features that differentiate CHS from other gastrointestinal disorders. As a first case report in Mexico, the authors consider this work is a good effort to recognize this syndrome. Nevertheless, the limitation of this paper consists in the lack of assessment throughout the time with the objective to obtain more clinical information on the prognosis and follow-up of the patient.
In addition, the CHS requires recognition and clinical consensus to develop algorithms for diagnosis and interdisciplinary treatment in order to achieve the only effective treatment for these patients, namely, the permanent cessation of cannabis use, and thus reduce the high costs derived from malpractice.
Finally, the increase in cannabis use worldwide requires specialists from various medical and paramedical specialties to update their knowledge on the toxic effects cannabis produces in the body to prepare assertive interdisciplinary prevention and treatment strategies at the three levels of the health system to counter public policies contemplating the legalization of cannabis for medicinal and recreational use in Mexico.