Introduction
Obsessive-compulsive disorder (OCD) is a neuropsychiatric disorder characterized by intrusive thoughts and/or repetitive behaviors that are time consuming and impairing (American Psychiatric Association, 2013; Sinopoli, Burton, Kronenberg, & Arnold, 2017). OCD is a heterogeneous disorder for which several subtypes have been identified. These subtypes are characterized by specific phenomenological, genetic, and neuro-anatomical features, and a different treatment response (Matsunaga, Maebayashi, & Kiriike, 2008).
These subtypes include the pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections (PANDAS), which is characterized by the onset or exacerbation of OCD symptoms and/or tics after a streptococcal infection (Cabrera-Mendoza, Genis-Mendoza, & Nicolini, 2019). Similar to Sydenham’s chorea, the hypothesized pathogenesis for PANDAS is an immune response against Group A beta-hemolytic streptococcal infections (GABHS).
It has been suggested that the serotonergic system is involved in OCD physiopathology (Bastani, Arora, & Meltzer, 1991; Nicolini, Cruz, Camarena, Páez, & de la Fuente, 1999; Margoob & Mushtaq, 2011). This hypothesis is supported by the therapeutic efficiency of selective serotonin reuptake inhibitors (SSRIs), which are the first line treatment for OCD (Billett et al., 1997). SSRIs act upon the serotonin transporter protein, encoded by the 5-HTT (SCL6A4) gene. The serotonin transporter (SERT) protein facilitates the uptake of serotonin released in the synaptic cleft after neurotransmission, terminating its action and therefore regulating its availability.
5-HTTLPR is a polymorphism found in the 5’ promoter region of 5-HTT and has two common alleles that correspond to an insertion of 43 bp (L allele) and its deletion (S allele). The S allele is characterized by a reduced transcription of the 5-HTT gene and lower functional activity than the L allele. Both alleles have been associated with OCD (Mak, Streiner, & Steiner, 2015; Hu et al., 2006; Bengel et al., 1999; Perez, Brown, Vrshek-Schallhorn, Johnson, & Joiner Jr, 2006; Lin, 2007; Hu et al., 2006). However, other studies have reported no association between 5-HTTLPR polymorphisms and OCD (Margoob & Mushtaq, 2011) probably due to OCD subtypes heterogeneity in the studied samples. Attending this lack of consistency, the stratification of OCD patients in homogeneous subgroups might clarify the role of this genetic variants in each OCD subtype (Sinopoli et al., 2017).
There is evidence suggesting that the immune system might play a role in the pathophysiology of OCD (for a review see Marazziti, Mucci, & Fontenelle, 2018). Serotonin has shown to be able to modulate a wide range of immune functions; for example, inflammation, phagocytosis, T cell migration, and cytokine production (Jaiswal, Mohanakumar, & Rajamma, 2015).
In patients with PANDAS, the presence of auto-antibodies has been reported providing evidence of the possible autoimmune etiology of this disorder (Nicolini et al., 2015). Genetic variants influencing serotonin availability, and consequently the immune response, as 5-HTTLPR polymorphisms, might be particularly relevant in PANDAS (Jaiswal et al., 2015). Therefore, the identification of genetic variants associated with auto-antibody production in patients with this OCD subtype is crucial to explain the pathophysiology underlying PANDAS.
This study aimed to determine whether there is an association between the L (long) and S (short) alleles of the 5-HTT gene and anti-streptococcus, anti-neural, and anti-enolase (anti-basal ganglia) antibody titers in patients with PANDAS from Mexico and Cuba.
Method
Study design
A cross-sectional design was used.
Subjects/description of the sample
Participants, including patients and controls, were recruited at the Adolescent Clinic in Havana, Cuba, and the Carracci Medical Group Clinic in Mexico City, Mexico during 2011. The presence of psychiatric disorders was evaluated with the MINI Modified International Neuropsychiatric Interview in adult participants, which is a short structured diagnostic interview for the 17 most common psychiatric disorders in DSM-IV and ICD-10 psychiatric disorders (Sheehan et al., 1998); meanwhile, this evaluation was performed in pediatric participants with the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID), which is the MINI interview adapted for children and adolescents (Sheehan et al., 2010). Patients with PANDAS had a history of recurrent streptococcal infections, OCD, and/or tics. OCD in PANDAS patients was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders IV edition criteria, while OCD symptoms severity was evaluated in adult patients by the Yale–Brown Obsessive Compulsive Scale (Y-BOCS) which is a self-report that consists of 10 items. OCD symptoms severity in pediatric patients was evaluated with the Children’s Yale-Brown Obsessive-Compulsive Scale (CY-BOCS), which is the children’s version of the Y-BOCS (Nicolini et al., 1996; Ulloa et al., 2004).
Participants in the control group obtained normal results in the applied questionnaires at enrollment, and did not have a history of recurrent infections, or neurological or psychiatric diseases. All participants were evaluated by a trained psychiatrist with about 20 years of experience. A consensus diagnosis was reached between psychiatrists of both recruitment centers for all participants.
Places
We randomly selected 56 patients diagnosed with PANDAS and 20 neurologically and psychiatrically healthy subjects who had been treated at the Adolescent Clinic in Havana, Cuba, or the Carracci Medical Group Clinic in Mexico City, Mexico.
Measurements: Antibody titration
The presence of the following antibodies in serum was determined by indirect ELISA: anti-neural tissue (brain glycoproteins lysate), anti-streptococcus (streptococcus protein lysate), and anti-neuronal enolase. Antigen obtention is described to detail in Nicolini et al. (2015).
Briefly, the antigens were diluted in buffer at a final concentration of 2 μg/ml. From this solution, 100 μl were added to each well (96-well plate) and incubated overnight at 4ºC. The following day, the wells were washed with .05% Tween/PBS, 120 μl of 5% skimmed milk diluted in .05% Tween/PBS were added and incubated 1 hour at 37ºC. The plate was washed once more with Tween/PBS, and 100 μl of diluted serum (1:100) were added and incubated for 2 hours at room temperature. Afterwards, the plate was washed once again with Tween/PBS and 100 μl of human HRP-conjugated IgG (1:10000) diluted in 1% skimmed milk and incubated for 1 hour at 37ºC. 100 μl of .1% o-phenylenediamine (diluted in 5mg OPA in 10 ml of citric acid/phosphate buffer).
Measurements: 5HTT genotyping
5HTT genotyping was performed by polymerase chain reaction (PCR) of genomic DNA (gDNA) extracted from peripheral blood. We determined the presence of three genotypes: LL, SL, and SS. The primers used were: forward 5-GGC GTT GCC GCT CTG AAT TGC and reverse 5- GAG GGA CTG AGC TGG ACA ACC CAC, amplifying the region within the 5HTTLPR gene containing the L or S polymorphisms (Gudayol-Ferré et al., 2010; Heils et al., 1996). The final reaction volume was 15 μL (1.5 mM MgCl2, .2 mM dATP, dCTP and dTTP, .1 mM dGTP and .1 mM 7-deaza-dGTP, 1 U Taq DNA polymerase, .25 μM primers, and 150 ng gDNA), and set under the following conditions: initial denaturation at 95ºC/2m, 40 cycles of 95ºC/30s, 62ºC/30s, 72ºC/1m, and final extension at 72ºC/5m. The PCR amplicons were resolved on 1.5% high-fusion agarose gels, the bands were stained with EZ-VisionTM (Ambresco) and visualized by UV light. The size of the alleles was compared against a 50 bp molecular weight ladder.
Statistical analyses
Statistical analyses was performed with the software SPSS 17.0 (SPSS Inc. Chicago IL, USA). First, we compared genotype frequencies and antibody titers between PANDAS patients and healthy controls separating samples from Mexico and Cuba. Then, PANDAS patients and controls from both countries were classified in three groups according to their genotype: LL, SL, and SS, and we performed comparisons of antibody titers among these groups. Descriptive statistics and t-tests for independent samples were used to describe and compare the titer antibodies differences between PANDAS patients and healthy controls for each genotype as well as within the PANDAS patients. The significance threshold was set at .05.
Ethical considerations
The present study was performed in accordance with the principles of the Helsinki Declaration in 1975. In addition, it was approved by the Ethics committees of the Medical Group Carracci in Mexico City, Mexico, and the Adolescent Clinic in Havana, Cuba. All the recruited patients or their legal representatives in the case of minors, provided their informed consent. In addition, minors provide their assent to participate in the present study.
Results
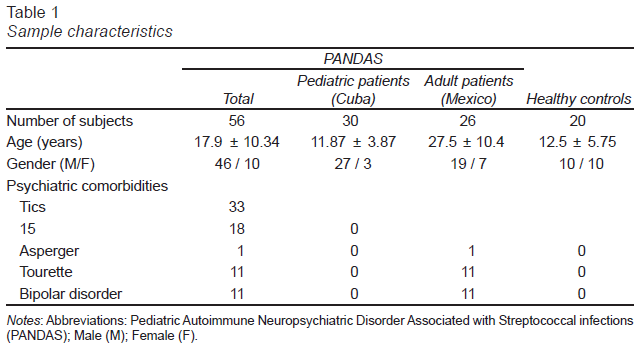
The sample was composed of 56 PANDAS patients and 20 healthy control. From the PANDAS group, 30 were Cuban and 26 Mexican. Most of the patients diagnosed with PANDAS were male (82%), which is consistent with previous reports in the literature (Swedo, Leonard, & Rapoport, 2004). We did not find significant differences in antibody titers when stratifying the data by gender.
The mean age for all the PANDAS patients, including Mexican and Cuban patients, was 17.9 years old and for the control group was 12.5 years old. Mexican patients had a significantly higher mean age than Cuban participants (p-value < .05). However, mean titers for the three evaluated antibodies were not different between Mexican and Cuban PANDAS patients. The sample characteristics are described in Table 1.
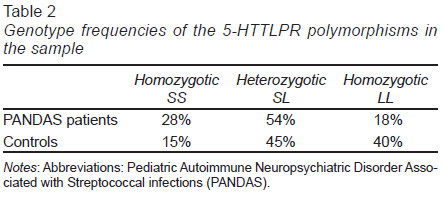
We observed a higher frequency of the SL and SS genotypes in patients with PANDAS relative to controls. Regarding the LL genotype, we did not detect a significant difference in its frequency between the groups. The genotype frequency found in PANDAS patients and controls is summarized in Table 2.
Separating by nationality, we found that the genotype frequency for SS and SL in Mexican PANDAS was 42% and 58%, respectively. All the Mexican controls presented the SL genotype (n = 3). According to our results, the LL genotype was absent in participants of Mexican origin (n = 19). Cuban PANDAS presented the following genotype frequency: 33% LL, 17% SS, and 50% SL. In the Cuban healthy controls, the genotype frequency was: 44% LL, 33.33% SL, and 22.22% SS.
When separating PANDAS patients and healthy controls from Mexico and Cuba, we found that Mexican PANDAS presented higher antibody titers for anti-enolase (p-value: .023), anti-neural (p-value: .003), and anti-streptococcus (p-value: .02), relative to healthy controls only in subjects with a SL genotype.
Regarding Cuban patients, PANDAS patients presented a significant increase in anti-streptococcus and anti-enolase titers in the SL (p = .02 and p = .019, respectively) and LL genotypes (p = .007 and p = .009, respectively), but not in anti-neural antibody levels compared to healthy controls.
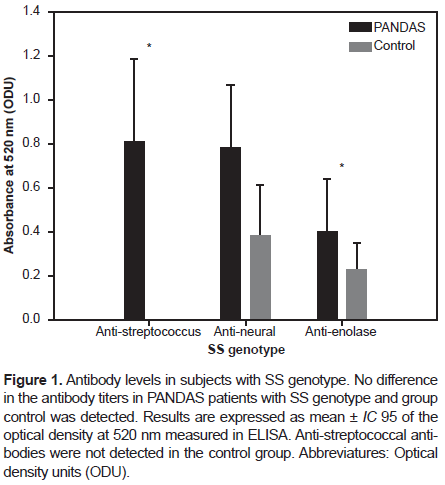
A joint analysis of PANDAS patients, comparing PANDAS patients from Cuba and Mexico to healthy controls from both countries, indicated a significant difference in the anti-streptococcus titers between PANDAS and healthy controls with the SS genotype (p-value: .04). No significant differences were found for anti-enolase and anti-neural titers between PANDAS and healthy controls with the SS genotype, as shown in Figure 1.
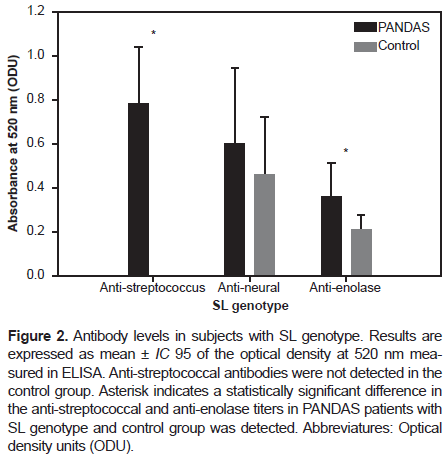
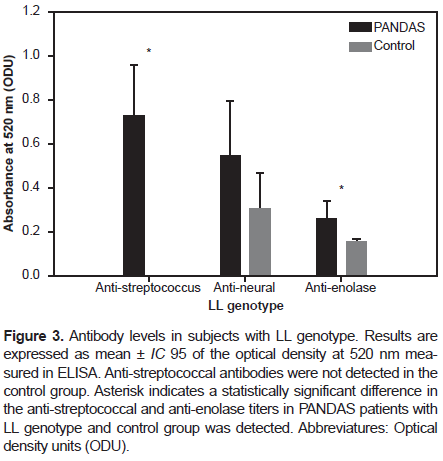
However, in participants with the SL genotype, we found a statistically significant difference in the anti-streptococcus and anti-enolase antibodies titers (p-value: .0017 and p-value: .0015, respectively) between PANDAS and healthy controls with the SL genotype (Figure 2). Regarding participants with the LL genotype, we found an even more statistically significant difference in the anti-streptococcus and anti-enolase antibodies titers (p-value < 0.001) between PANDAS and healthy controls, as shown in Figure 3.
Discussion and conclusion
Neuropsychiatric disorders have been linked to immune system and serotonin signaling abnormalities (Marazziti et al., 2018). The alteration in the interaction between both systems may contribute to behavioral disorders. It has been suggested that serotonin exerts complex regulatory effects on the multiple immune cells where its transporter and receptors are expressed, although the function of these molecules in the immune system is not fully understood (O’Connell et al., 2006).
Since an autoimmune basis has been suggested for PANDAS, genetic variants that could influence behavior and immune response are potentially relevant in the elucidation of its etiology. To the best of our knowledge, this is the first study exploring the association between the 5-HTTLPR genotype and antibody titers against streptococcal, basal ganglia (i.e. anti-gamma enolase), and neural tissue in PANDAS patients.
The study of antibody titers in OCD patients by Nicholson et al. (2012) reported a higher incidence of positivity for anti-basal ganglia in OCD individuals compared to psychiatric controls (depression and schizophrenia). Most positive sera had antibodies against the enolase antigen. No differences were reported in anti-streptolysine-O antibody titers between OCD subjects and controls; in addition, no association between the presence of anti-streptolysine-O and anti-basal ganglia antibodies was identified. Our results contrast with such report, as we detected higher anti-streptococcal antibody titers in patients with PANDAS relative to healthy controls, irrespective of the genotype.
Beside the serotonin transporter polymorphisms, the effects of polymorphisms in other genes involved in serotonergic neurotransmission and immune response could provide valuable information about the interaction between both systems (Mössner & Lesch, 1998; Ahern, 2011). Some of these genotypes have been studied in related disorders; for example, the expression of the allele LA in the promoter region of the 5-HTTLPR gene showed a higher prevalence in patients with Gilles de la Tourette syndrome with no OCD (Moya et al., 2013). The SERT I425V polymorphism, which causes a variation in the aminoacid sequence of the protein, induces a higher serotonergic activity and has been associated to OCD and Gilles de la Tourette syndrome (Zhang, Gesmonde, Ramamoorthy, & Rudnick, 2007; Moya et al., 2013).
The latter suggests that, functionally, the presence of other serotonergic polymorphisms could be regulated differentially at mRNA level, promoting a higher serotonin transport activity and thus explaining the symptoms exhibited by OCD and Gilles de la Tourette patients. In our study, the presence of an LL genotype was associated to increased titers of the tested antibodies in comparison to the other genotypes (SS and SL). As mentioned previously, the L and the S alleles are characterized by an increased and reduced transporter activity, respectively. An increased or decreased activity in serotonin transport cannot be inferred from our results and we can only state that higher antibody titers were observed in those patients carrying one or two L alleles. Patients with L allele represented 33% of the cases among the Cuban subjects and 42% of the Mexican subjects in our sample.
A meta-analysis of the S allele did not demonstrate a clear association between this allele and OCD; however, when meta-analysis data were stratified by gender, it revealed an association between the allele S and OCD in females (Mak et al., 2015). When we stratified our data by gender, the statistical analysis of antibody titers did not reveal a significant difference between the tested genotypes and the controls in our study. Apparently, the increased titers of the tested antibodies are not associated to the S genotype but only to L genotype, increasing the evidence for OCD heterogeneity.
Despite the lack of Mexican participants with a LL genotype in our sample, previous reports have demonstrated the presence of this genotype in the Mexican population. A previous association study of 5-HTTLPR in Mexican patients with OCD, the frequency of the genotypes was of SS = 32%, SL = 51%, and LL = 17% (Camarena et al., 2001), although it should be clarified that the patients from such study were adults with a diagnosis of OCD, without apparent characteristics of PANDAS.
Since OCD is a heterogeneous disorder, its etiology could not be explained by a single gene but rather by a polygenic basis, as well as environmental factors as child abuse and daily life stress in which the 5-HTTLPR genotype could act as a moderator in the development of a psychiatric disease (Caspi et al., 2003; Caspi et al., 2008). It is proposed that the S allele moderates the influence of life stressing events in the onset of depressive symptoms and suicide risk when compared with homozygous carriers of the allele L (Caspi et al., 2003; Contreras et al., 2010), for which our study found an associated increase in autoantibody titers.
A recent study reported the increment of anti-neural antibodies, such as anti-lysoganglioside GM1 and anti-dopaminergic receptors DR1 and DR2, and distinguished PANDAS patients with and without choreiform movements (Singer et al., 2015). Therefore, antibody measurements in patients with PANDAS could not only shed light on the etiology of this disorder, but, also be very useful in therapeutic follow-up and prognosis as antibody levels are correlated with symptoms.
Mexican patients carrying an SL genotype showed a significant difference between the levels of anti-neural antibodies compared with the controls from Cuban origin, in which this was not observed. Other causes, including polymorphisms in other genes, that could be related to ethnicity and population, should be addressed in other studies.
Limitations of the present study should be acknowledged. First, the sample size was relatively small. Thus, future research with larger samples is required to replicate our results. Second, our sample was highly heterogeneous in terms of ethnic background, age, and comorbid psychiatric disorders. For example, participants were recruited in two different countries, Mexico and Cuba, that may present different genetic composition as well as different environmental factors that might influence antibodies titers.
Also, participants recruited in Cuba were younger than those recruited in Mexico City. Although we did not find significant differences in antibody titers between participants from both countries, age may represent a confounding factor that should be addressed in future research by its inclusion during statistical analysis or sample stratification. PANDAS patients had several psychiatric comorbidities, including tics, Asperger, Tourette, and bipolar disorder. Future studies should evaluate the effect of mental disorders, including an adequate number of participants with such mental comorbidities to estimate this effect.
This study suggests an association between the analyzed polymorphisms in the promoter region of the 5-HTTLPR gene and the increased titers of anti-neural, anti-enolase, and anti-streptococcal antibodies in the serum of PANDAS patients, and the early onset of this disorder. Although said association is not yet fully clear, antibody titers measurements could be used as a diagnostic tool for different OCD subtypes, and other related disorders, in clinical practice, being potentially relevant for the implementation of personalized treatment (Orefici, Cardona, Cox, & Cunningham, 2016).